From our headquarters in Toronto, Ontario, we develop biotechnological formulations that meet the highest international standards


At TrueNorth Labs (TNL), we believe that trust is built on quality, compliance, and transparency.
From our headquarters in Toronto, Ontario, we develop biotechnological formulations that meet the highest international standards.
TrueNorth Labs is a Canadian company specializing in biotechnology and pharmaceutical solutions. We source raw materials from strategic international suppliers (China, India, Europe, and the U.S.), which then undergo validation and quality control processes under Canadian standards. This allows us to offer our customers a cost-competitive product with the safety and traceability demanded by the international market.
Our purpose is clear: to be a trusted partner for clinics, institutions, and healthcare professionals, offering stable, traceable products backed by scientific evidence.
TNL isn’t just a name: it’s a seal of trust.
Each presentation reflects our commitment to responsible innovation and rigorous quality control.
Choosing TNL means working with a laboratory that understands the demands of the industry and responds with clear, safe, and consistent solutions.

Each development is designed under strict laboratory standards, ensuring stability, traceability, and regulatory compliance.
It is an innovative molecule that acts as a dual agonist of GLP-1 and GIP, internationally approved for the management of type 2 diabetes, with additional benefits in weight control. It regulates glucose by stimulating insulin, reducing glucagon, and generating prolonged satiety.
- More effective glycemic control.
- Support for weight loss as an associated effect.
- Backed by solid clinical evidence and regulatory approval.
- Lyophilized Vial – 120 mg, 90 mg → Sterile powder for reconstitution at the time of use, ensuring stability, purity, and traceability. Other strengths can be developed upon institutional request and technical validation.
- Multi-dose Prefilled Pen – 60 mg → Multi-use prefilled device that simplifies clinical practice, offering speed, efficiency, and consistent dosing.
Other strengths can be developed upon institutional request and technical validation.

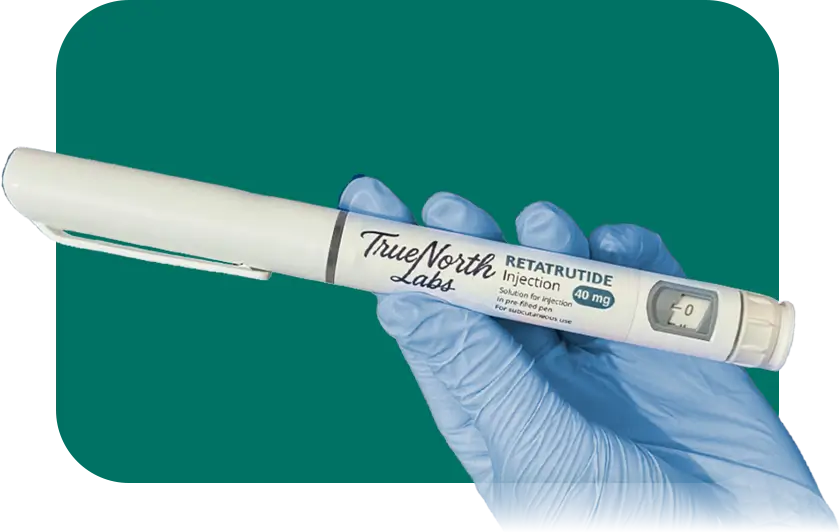
It is a triple agonist (GLP-1 + GIP + glucagon) in advanced clinical trials and is considered the next generation of metabolic therapies. It activates three hormonal pathways, increasing energy expenditure, optimizing glucose regulation, and enhancing fat oxidation.
- Superior weight loss potential compared to current molecules.
- Greater impact on comprehensive metabolic control.
- Projected as a future benchmark in cardiometabolic therapies.
- Lyophilized Vial – 40 mg → Sterile powder formulation designed for protocols requiring precision and detailed control.
Other strengths can be developed upon institutional request and technical validation.
- Multi-dose Prefilled Pen – 40 mg → Practical and reliable device for repeated applications, offering accurate dosing and operational efficiency.
Other strengths can be developed upon institutional request and technical validation.
It is a triple agonist (GLP-1 + GIP + glucagon) in advanced clinical trials and is considered the next generation of metabolic therapies. It activates three hormonal pathways, increasing energy expenditure, optimizing glucose regulation, and enhancing fat oxidation.
- Superior weight loss potential compared to current molecules.
- Greater impact on comprehensive metabolic control.
- Projected as a future benchmark in cardiometabolic therapies.
- Lyophilized Vial – 40 mg → Sterile powder formulation designed for protocols requiring precision and detailed control.
Other strengths can be developed upon institutional request and technical validation.
- Multi-dose Prefilled Pen – 40 mg → Practical and reliable device for repeated applications, offering accurate dosing and operational efficiency.
Other strengths can be developed upon institutional request and technical validation.

• HGH 191AA (Somatropin)
• IGF-1 LR3 / IGF-1 DES
• MGF / PEG-MGF
• GHRP-2 / GHRP-6 / Hexarelin
• Ipamorelin
• CJC-1295 (con y sin DAC)
• CJC-1295 + Ipamorelin
Our processes comply with:
- Good Manufacturing Practices (GMP).
- ISO quality control protocols.
- Full traceability for each batch.
This ensures that each TNL product reaches institutions with the highest guarantee of safety and consistency.
At TNL, we follow a rigorous control model:
1. Controlled production under international standards.
2. Internal and external validation of each batch.
3. Release with technical documentation for institutions.
4. Continuous support for our partners in each market.
Our vision is to establish ourselves as a benchmark in biotechnology applied to healthcare, providing solutions that combine innovation, safety, and compliance.
Our commitment is to the trust of institutions and the well-being of patients, always through traceable, reliable products developed under international standards.
At TNL, compliance isn't a requirement: it's part of our identity.
- Our products are intended exclusively for licensed institutions and professionals.
- We do not sell directly to the public or issue therapeutic recommendations.
- All information on this site is for strictly institutional purposes.
Would you like to receive technical sheets, catalogs, or information for institutional integration?